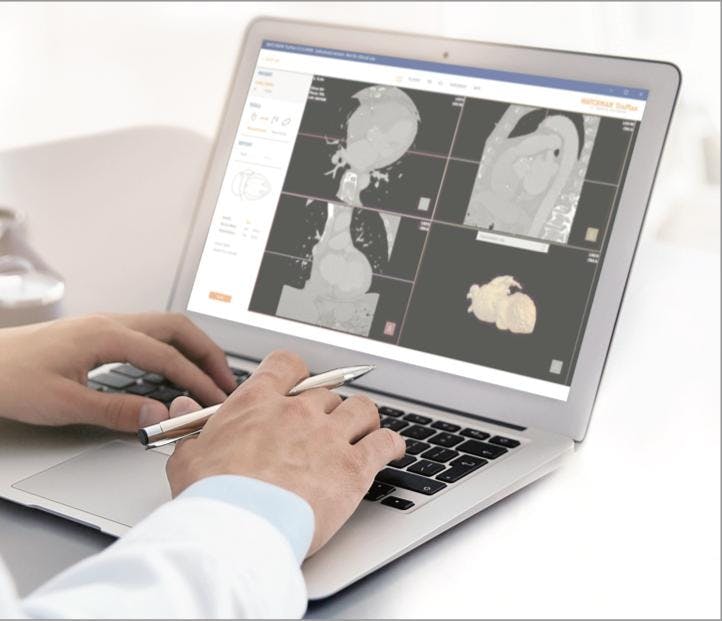
WATCHMAN TruPlan Software is a purpose-built CT imaging software that includes comprehensive tools to guide your LAAC clinical decisions more precisely.

v3.3.0 FDA/Health Canada/TGA for US, Canadian, Australian and EU Customers
Clearances
Archives
Support
Support phone: +1 (403) 338 1870 (8am-5pm Mountain Time)
Support email: support@circlecvi.com
Regulatory
Require further information on Circle's Quality System or the regulatory approval status for Circle products?
Compliance Information
Circle Cardiovascular Imaging Inc. holds globally recognized ISO 13485:2016 and MDSAP certificates from the prestigious British Standards Institution (BSI). In addition to ISO 13485:2016 and MDSAP, the company quality system is established and maintained to comply with European Medical Device Directive 93/42/EEC, European Medical Device Regulation 2017/745, Australian Therapeutic Goods (Medical Devices) Regulations, US FDA Quality System Regulations 21 CFR Part 820, and other global medical device regulations.
Circle’s software development process incorporates several globally-recognized standards and guidances, including: BS EN (IEC) 62304:2006+A1:2015, BS EN (IEC) 62366-1:2015+A1:2020, and IEC 82304-1:2016. An intensive risk management process, based on BS EN (ISO) 14971:2019+A11:2021, is also implemented as part of the development process, to capture the potential harms, hazards, and hazardous situations, and to mitigate these identified risks.
If you require further information on Circle's Quality System or the regulatory approval status for Circle products, please contact the following:
Dr. Shirantha Samarappuli
Vice President
Regulatory Affairs and QMS
Email: shirantha.samarappuli@circlecvi.com
Phone: +1 (587) 747-4692
BSI ISO 13485:2016 Certificate # FM 539204 (Effective: Aug 31st, 2024)
BSI ISO 13485:2016 Certificate # MDSAP 689664 (Effective: Aug 31st, 2024)